Summary of Research Interests and Accomplishments
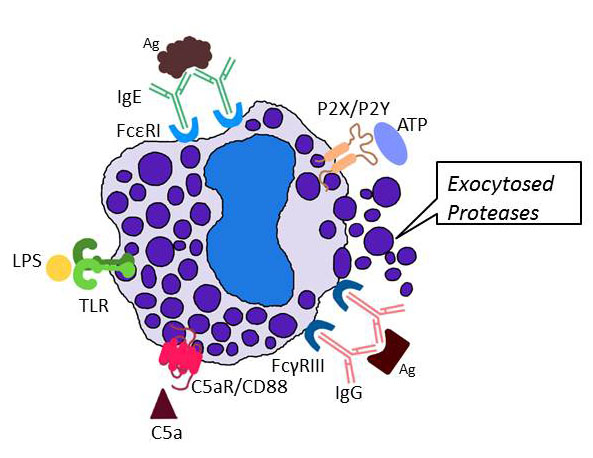
My research program is focused on the biochemistry, molecular biology, and cell biology of mast cells (MCs) and other effector cells that participate in blood coagulation, inflammation, connective tissue remodeling, acquired immunity, and innate immunity. In regard to MCs, we and others showed that these tissue-resident immune cells release a diverse array of biologically active molecules (including varied cytokines, chemokines, leukotrienes, prostaglandins, histamine, serglycin proteoglycans, carboxypeptidase, tryptases, chymases, and elastases) when activated via their high-affinity IgE, low-affinity IgG, complement, purinergic/ADP, and protease-activated receptors (see Figure 1) .
Fig. 1. Exocytosis of neutral proteases from MCs. When activating receptors on the surface of a tissue MC encounter the appropriate ligand, the cell quickly releases the contents of its secretory granules which are predominantly protease-serglycin proteoglycan complexes (from Douaiher et al., Adv. Immunol. 2014;122:211-251).
As noted at Research Gate (http://www.researchgate.net/) and Google Scholar (http://scholar.google.com/), I am a leader in the MC field, and my peer-reviewed scientific publications highlighted at Google Scholar have been cited >14,000 times at a rate of ~300/year for the last three decades with ~80 citations/publication. For example, it is now known that mouse MCs store varied combinations of 16 neutral proteases in their secretory granules ionically bound to serglycin proteoglycans that contain chondroitin sulfate E and/or heparin glycosaminoglycans. We cloned the genes and transcripts that encode mouse, rat, and human serglycin, identified chondroitin sulfate E glycosaminoglycans in mouse and human MCs, and created a heparin-null mouse by targeted inactivation of the gene that encodes the biosynthetic enzyme N‑deacetylase/N‑sulfotransferase. In addition, nearly all of the neutral proteases ionically bound to these intracellular proteoglycans of mouse MCs were initially identified and cloned by my group, as well as some of their human orthologs.
We recently discovered that sea squirts contain MCs that morphologically and biochemically resemble those in human skin. It therefore is apparent that the constitutive MCs that reside in our connective tissues are among the most fundamental cells of our immune system because they evolved >500 million years ago before the appearance of B cells, T cells, and adaptive immunity.
Paul Ehrlich first discovered MCs in 1878 based on histochemical criteria. Since that time, no human has been found that lacks MCs, and we showed that the cell’s tetramer-forming tryptases are needed to prevent the internal accumulation of life-threatening fibrin deposits and fibrin-platelet clots by proteolytically destroying fibrinogen before this plasma protein can be converted to fibrin by thrombin.
MCs play central roles in numerous inflammatory disorders. W/Wv and Sl/Sld mice constitutively have reduced numbers of MCs in their tissues due to genetic defects in the genes that encode the tyrosine kinase receptor Kit/CD117 and its ligand, respectively. Studies carried out on these mice revealed that MCs are beneficial in bacterial and helminth infections, and we showed that the tetramer-forming tryptases exocytosed from activated MCs are needed for combating Klebsiella pneumoniae infections effectively. Our collaborators and I were the first to show that human MCs and their progenitors are highly susceptible to M‑tropic strains of HIV-1. Thus, it is now generally accepted that the loss of HIV-1-infected MCs in varied tissues of AIDS patients contribute to their inability to combat opportunistic infections. In support of this conclusion, transgenic mice that lack MC-restricted tetramer-forming tryptases have a diminished ability to combat pneumovirus infections of their lungs.
Despite the beneficial roles of MCs and their protease mediators in blood coagulation and varied infections, the presence of increased numbers of activated MCs in the skin of mastocytoma patients, in the bronchial airways of individuals with asthma, chronic allergies, and chronic obstructive pulmonary disease (COPD); in the skin of patients with systemic mastocytosis or undergoing extensive fibrosis; in the gastrointestinal tract of helminth‑infected individuals and patients with inflammatory bowel disease (IBD); in tumors of cancer patients; and in the joints of those afflicted with rheumatoid arthritis suggest that these effector cells contribute substantially to the pathology that occurs in numerous serious diseases. In this regard, my coworkers and I showed that MC‑restricted tryptase•heparin complexes play key roles in experimental arthritis, ulcerative colitis, heart disease, and COPD, in part, by inducing bystander cells in tissues to markedly increase their expression of those chemokines that induce the accumulation of large numbers of neutrophils and other immune cells into inflamed tissues. Some of these exocytosed MC proteases also play significant roles in connective tissue remodeling by activating matrix metalloproteinase (MMP) zymogens. The importance of the MC’s tryptases in blood coagulation, inflammation, connective tissue remodeling, innate immunity, and acquired immunity were previously missed due to a failure to appreciate their redundant, overlapping bioactivities. In that regard, we discovered that one must knock out at least two of the genes that encode the three tryptases present in mouse and human MCs in order to appreciate the beneficial roles of this family of serine proteases in preventing the internal accumulation of fibrin deposits and fibrin-platelet clots, as well as their adverse roles in experimental arthritis, IBD, and COPD.
In regard to the MC’s chymases and elastases, we showed that this distinct chromosome‑14 family of serine proteases play key roles in the wound-repair processes that occur in arthritis, thermal injury, and ischemia‑reperfusion injury.
My group showed in the 1980s and 1990s that mammalian MCs are a heterogeneous family of hematopoietic cells whose ultimate phenotype is dependent on the tissue microenvironment the mature cell eventually resides. Due to the ability of MCs to reversibly alter their phenotype and due to their long half‑life in tissues, there might not be two MCs in the body that are identical. We developed varied in vitro and molecular biology approaches to identify the factors and mechanisms that regulate the differentiation and phenotypic properties of mature MCs in mice and humans. For example, we identified a novel calcium-regulated guanine exchange factor/phorbol ester receptor (designated as RasGRP4) that determines which cytokines and chemokines are released from activated MCs. By varying the culture conditions, we induced bone marrow progenitor cells to differentiate and mature into populations of MCs that express different proteoglycan, protease, and lipid mediators. In that regard, the in vitro systems that my coworkers and I developed in the 1980s are still used today by many to generate different populations of non-transformed mouse MCs.
We identified a number of novel human and mouse MC-specific genes. We are now investigating how these genes and their transcripts are regulated as the MC’s microenvironment is altered. Cis-acting elements and trans-acting DNA-binding proteins that regulate the transcription of these genes, and the RNA-binding proteins and miRNAs that regulate the stability and half-lives of their transcripts are being identified.
We created transgenic mice that differ substantially in the number of MCs they have in their tissues. Other transgenic mice have been created that lack RasGRP4 and numerous MC-restricted granule mediators. A transgenic/adoptive transfer approach using newly created v‑abl-immortalized MCs also has been developed to help evaluate the roles of MCs and their different mediators in mammals. In addition, recombinant MC proteases have been generated in order to deduce their physiologic function. Finally, what happens to T cells, epithelial cells, endothelial cells, fibroblasts, chondrocytes, smooth muscle cells, neurons, and macrophages when they interact with MCs and their granule protease mediators are being investigated.
The importance of MCs and their tryptase mediators in blood coagulation, sepsis, arthritis, COPD, and IBD are highlighted in greater detail below to better document the importance of the work being carried out by my group in collaboration with others throughout the world. In regard to experimental arthritis, IBD, and COPD, the factors and mechanisms that initiate these diseases are very different. Nevertheless, our mouse data suggest that the late‑stage, effector phases of these inflammatory diseases are similar in terms of their dependence on MCs and their exocytosed protease heparin complexes.

Comments are closed.